Senior Design - External Female Catheters
August 2021 - Present
Senior Design is a two-semester course used to develop a prototype per the FDA's Quality System Regulation, 21 CFR 820, with a focus on subsection 820.30. I worked with a team of seven engineering students to develop a new external female catheter design, utilizing ethnography, user needs, and hazard and risk analysis to create prototype specifications.
This portfolio focuses on individual, design-related contributions to the project. The second semester will involve verification and validation of the frozen prototype.
Ideation
NEED FOR PROJECT
Unmet clinical needs were explored by speaking with doctors, nurses, and other healthcare providers. External female catheters were chosen as a project due to ethnographic data showing a ubiquitous need for a more comfortable, mobile, and accurate device. They also can greatly reduce catheter-associated urinary tract infections compared to indwelling catheters.

OBJECTIVES
My responsibilities were to contribute to design specifications and prototyping efforts, as well as handling submission to the University of Pittsburgh's Institutional Review Board (IRB) for human subjects research. I also took over as team leader in the last month, ensuring that deadlines were met and tasks were distributed appropriately.
Prototyping
Initial Efforts
I developed the idea of attaching the existing PureWick external female catheter to underwear to reduce movement. A clip on either end of the device pushes through underwear to secure it in place. Initial prototypes were made of modeling clay.




A hole was added to the underwear to fit suction tubing from the device. To ensure proper hole placement, a real PureWick was aligned with underwear on a female pelvic model.
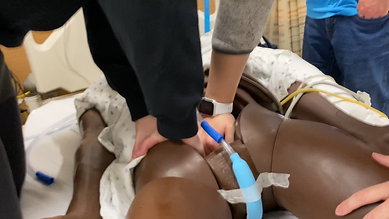
Flow Rate
To match normal urination of 18mL/second, suction rate testing was conducted on a PureWick using both a fish tank pump and vacuum. Different lengths and sizes of tubing were optimized.



Tubing
Tubing holes were added to increase suction, decreasing leakage and improving measurement accuracy. Different hole patterns were tested in a nursing skills lab with a manikin and wall suction. I timed the trials and collected data.

Fusion Modeling
To improve device comfort, other team members generated a shorter, wider PureWick model in Fusion. The model will be used to mold and cast a rubber shell next semester.

Other Responsibilities
IRB
I took sole responsibility for submitting a human subject research plan to the institutional review board (IRB). Due to time constraints of the course, it was decided that we would interview healthcare providers about the prototype rather than apply for an investigational device exemption (IDE) through the FDA.


Team Leader
During the last month of our first semester, I took over as team leader. The team leader was responsible for ensuring deadlines were met, distributing tasks on a Gantt chart, and keeping the team on track for prototype completion. In addition, I will be responsible for an electronic quality management system (eQMS) to manage verification and validation next semester.
Verification and Validation

Team Leader
I continued my role as team leader. Responsibilities included:
-
Distributing tasks and ensuring deadlines were met.
-
Reviewing and approving all controlled documents unless I was an author.
-
Overseeing V&V progress.
Frozen Prototype





Wicking Speed
Wicking speed was tested using the modified external female catheter to ensure proper suction. I wrote the protocol and first of two reports.
Other team members tested leakage, mobility, and physical specifications.

Engineering Change Order (ECO)
Excess leakage necessitated an ECO investigation. I wrote a protocol and report for new tubing hole designs. Ultimately, a protocol change was needed instead of a new design.
Validation
Testing the modified PureWick on humans would have required FDA approval, which was outside the scope of our course. Instead, subjects that worked with external female catheters were interviewed to assess efficacy of the new design.



